Greenhouse
Gases and Earth’s Energy Balance
1. The Meaning of Temperature and
Energy
2. The Temperature of the Early
Earth
A. Simple Calculation of Early Earth
Temperature
B. More Complicated (but more
accurate) Calculation of Early Earth Temperature.
3. The Greenhouse Effect and Natural
Greenhouse Gases
Global Warming Potential of
Greenhouse Gases and the Energy (Im)Balance of Our Current Atmosphere
Earth’s Energy Balance
1. The Meaning of Temperature and Energy
Temperature
We have
discussed temperature in this course without defining what we mean. It's hard
to spend a winter in Michigan without having an intuitive feel for temperature,
but to understand planetary temperatures and greenhouse warming we need a more
precise physical meaning.
All matter (gases, liquids and solids) is made
up of atoms in various chemical relationships. Atoms consist of a nucleus
orbited by electrons, and are extremely tiny; there are about
10,000,000,000,000 atoms in a cubic centimeter! Because the
numbers are so large it usually makes sense to talk about average properties.
|
|
|
|
|
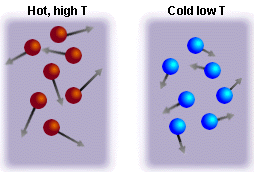
Figure 1. In a hot body, the
atoms move rapidly (individual atoms have different speeds, but the mean
speed is high). In a cold body, the atoms have relatively low speeds. If
collisions occur between hot and cold atoms, the average speed of the atoms
(and therefore the temperature) would be intermediate between the high and
low cases. |
Atoms
move randomly, jiggling furiously (at speeds of tens of kilometers per second)
and continually colliding with each other. When a cold object (such as a hand)
comes into contact with a hot object (say a hot kettle) energy is transferred
by these atomic collisions from the hot to the cold body. The energy flows from
hot to cold because the atoms in the cold body are pushed to greater speeds
(see Figure 1).
Temperature is a measure of the average speed
of the moving atoms. The faster they jiggle, the higher the temperature. The
mathematical definition tells us that temperature increases as the (mean atomic
speed)2.
Mathematically we say: ![]() ,
where v is the average atomic speed.
,
where v is the average atomic speed.
Temperature is measured in units called
degrees. For science, the most useful scale is the Kelvin scale. The Kelvin
scale starts from a value of absolute zero--a temperature corresponds to
perfectly stationary atoms (a situation that cannot be attained in practice). Each
degree Kelvin is equivalent to one degree Celsius, with 273K equal to 0oC).
The Earth's mean surface temperature is about 300K.
Energy
There are several types of energy. Some are :
- Kinetic energy: energy associated with
motion
- Potential energy: energy associated with
position
- Internal energy: energy associated with
atomic speed
- Radiant energy: energy associated with
radiation
- Chemical energy: energy associated with
chemical bonds
Energy has to be conserved; it can change
forms but the total amount must remain the same. We cannot create or destroy
energy. This is the famous Conservation
of Energy Principle.
2. The Temperature of the Early Earth
Let's calculate the temperature of the early
Earth. The initial hydrogen and helium have probably been lost through
gravitational escape processes and the atmosphere as we know it today has not
yet evolved. We are left with a planet that is cold and airless. We can
calculate the temperatures expected for airless planets warmed by the Sun's
rays in two ways. First, let's use a radiation law known as the "R-squared" Law.
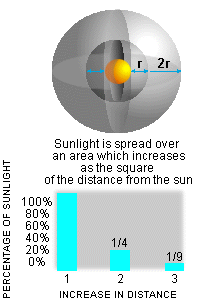
The R-Squared law states that the farther you
are from an emitting object, the less light you receive. In fact, a doubling of
the distance away reduces radiation by a factor of four. This is best explained
with a picture (see Figure 2).
We expect planets farther from the Sun to be
colder. The R-squared model allows us to calculate how much colder each
successive planet would be, based on an estimate of the amount of light
received. We can use this law to calculate the temperatures of each planet
using the current-day temperature of Mercury as a reference.
|
Figure
2. R-squared Law. |
A. Simple Calculation of Early
Earth Temperature
|
|
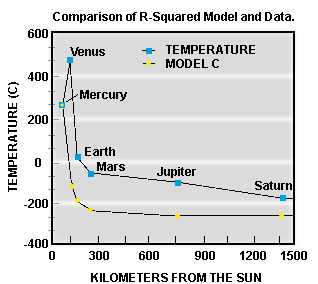
If we
assume that Mercury's temperature has not changed, we can calculate the
reduction in energy received by each planet using the R-Squared Law, knowing
only its mean distance from the Sun.
Figure 3 shows the results of this
calculation compared with the actual current day temperatures of the planets.
We can see that the simple model does not work very well. Venus is much, much
hotter than we would expect. Earth, Mars, Jupiter and Saturn are also hotter
than expected - what is going on??
Let's see
if we can get a more accurate model of the temperature of Early Earth. This
model is slightly more complicated. (You will not be examined on the
following).
B. More Complicated (but more
accurate) Calculation of Early Earth Temperature.
The
principle of Conservation of Energy tells us that the energy from the Sun
absorbed by a planet must equal the energy lost by the planet. Since the planet
floats in space, the only way to add or subtract energy is through radiation.
Therefore we can say for the Earth:
The amount of energy radiated to space from
the Early Earth depends on its temperature. Temperature can be calculated using
the Stefan-Boltzmann Law. Let's go through the
simple arithmetic to calculate the temperature of the Early Earth.
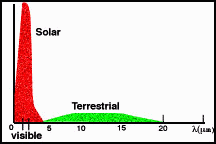
First, recall the types of radiation that the
Sun and Earth emit (Figure 4).
|
|
Figure 4. Solar and Terrestrial Radiation. |
The Sun
emits a lot of light in the visible range of wavelengths. We assume that the
portion of this light that hits the Earth is absorbed. The Early Earth emits in
the infrared. We assume that all of this radiation is lost to space.
The Law of Conservation of Energy
tells us that the amount lost (in the infrared) has to equal the amount
received (in the visible). Another way of viewing this is to say that the
temperature of the Early Earth has to rise until exactly as much energy is lost
through radiation as is gained from solar absorption. This equilibrium sets the
temperature of any atmosphereless planet.
To
calculate the temperature, we need to know how much light is received by the
Earth and how much is lost through emission. Let's do this in easy steps.
Step 1.
According to the Stefan-Boltzmann
Law, the amount of light emitted by the Sun =![]() ,
,
where Ts is the temperature of the solar surface (assume 6000°K).
Step 2.
The amount of this light that is absorbed by the Earth can
be determined if we recognize that the sunlight is "diluted" by the
time it reaches the Earth's orbit by a factor of ![]() where
Rs is the radius of the Sun and rs is the Earth-Sun distance. This dilution is
due to the "R-squared" law discussed above.
where
Rs is the radius of the Sun and rs is the Earth-Sun distance. This dilution is
due to the "R-squared" law discussed above.
Therefore, the light received by the Earth is given by: ![]()
where re is the radius of
the earth. (Energy input = amount emitted by the Sun multiplied by the
R-squared dilution factor and by the area cut out of the Sun's radiation beam
by the earth) (see Figure 5)
|
Figure 5. The amount of sunlight
falling on the Earth per square centimeter is given by multiplying the
intensity at the Earth's orbit by the area cut out of the Sun's radiation
beam by the Earth. |
Step 3.
We can also write down the amount of radiative energy lost
from the Earth, again using the Stefan-Boltzmann law: ![]() ,
where Te is the temperature of the Early Earth. (Energy output =
amount emitted per unit area of the Earth multiplied by the surface area of the
Earth)
,
where Te is the temperature of the Early Earth. (Energy output =
amount emitted per unit area of the Earth multiplied by the surface area of the
Earth)
Step 4.
Using the Law of Conservation of Energy, equate energy
input to energy output and solve the equation for Te: ![]()
(Energy input = Energy output)
After cancellation: 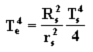
Step 5.
Calculate Te.
For the Early Earth Te = 283°K.
Step 6.
This calculation assumes that all the sunlight falling on
the Early Earth was absorbed. If we assume that some of it (say 83%, like
modern day Mars) is reflected, the temperature is 260oK. This is
about 40o colder than the temperature today - for reasons to be
explained below.
We have just
calculated the Radiative Equilibrium
Temperature of the Earth. It is the temperature that the Earth
would have with no atmosphere, when infrared emission exactly balances the
radiation received by the Sun.
But, we know that
our actual temperature today is ~300°K. What is wrong with our calculation? The
atmosphere is responsible for increasing the actual temperature above the
radiative equilibrium temperature. This increase is the so-called Greenhouse
Effect.
3. The Greenhouse Effect and Natural Greenhouse Gases
|
|
|
|
|
Figure 7: Simple picture of
Radiative Transfer |
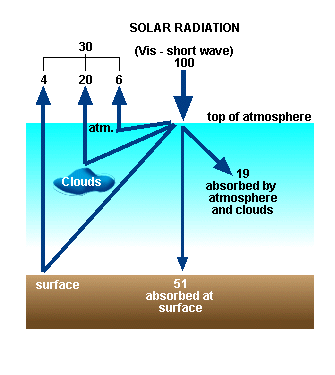
Shortwave Radiation Budget
Results
from a more sophisticated calculation than the one described previously demonstrate the magnitude of the
natural Greenhouse Effect for the planets (see Figure 6).
The natural
greenhouse effect is responsible for life as we know it, and should
be distinguished from the infamous anthropogenic greenhouse effect that is
currently causing so much concern. The natural Greenhouse Effect is beneficial
and warms our planet to more livable temperatures.
Why are the planets warmer than expected on
the basis of theory? The answer is that certain gases in the atmospheres of
these planets act to warm them up. The explanation is best understood by
reference again to the type of radiation emitted by the Sun and by the planets
(see Figure 7, below). Note that we need an extra 70% leaving the top to
achieve equilibrium. Where does it come from?
Over a long term average, the Earth and its
atmosphere must radiate as much energy out to space as it receives from the
sun. In fact, the same type of balance exists between the Earth and its
atmosphere!
Albedo (L) = percentage of incoming radiation
that is reflected back into space = 30% for Earth
(higher for Venus)
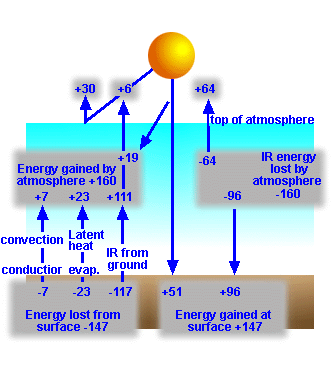
Longwave Radiation Budget
Now let's look at the long wave (IR)
component of the planetary radiation budget (Figure 8):
|
|
|
|
|
Figure 8:
Infrared component of the planetary radiation budget |
At the Earth's surface, we note that the gain and loss in energy is greater
than that received from the Sun - how can this be?
|
Earth's Surface |
|||
Gains
|
Losses
|
||
|
51 |
Visible
from Sun |
7 |
Conduction,
Convection |
|
96 |
IR from
atmosphere |
23 |
Evaporation |
|
|
|
117 |
IR
radiation |
|
147 |
net |
147 |
net |
The answer makes sense when we consider that
the surface of a planet receives a great deal of energy from its own
atmosphere. Thus the effect of the atmosphere is to warm the surface over the
temperature above that resulting from the Sun's energy.
We then have to ask, how does the atmosphere increase the
Earth's temperature?
The atmosphere warms the Earth by
"trapping" radiation, allowing the surface to warm to 300K. At that
temperature, the black body surface radiation is large enough to ensure that an
equilibrium condition pertains. The atmosphere traps radiation through the
action of certain gases, called Greenhouse Gases. These gases (e.g., CO2,
H2O, NO, CFCs, CO) are very good at absorbing and re-emitting
infrared radiation. They intercept the IR radiation from the ground and reflect
some of the energy back to the ground, warming it up more than would occur
otherwise.
The Greenhouse Effect provides additional
heat!
Summary
- Temperature is a measure of the square of the mean
speed of atoms and molecules.
- Energy comes in different types, but must be
conserved as a whole.
- The temperature of the early Earth (and other airless
planets and satellites) was determined by the balance between the
absorption of solar visible light and the emission of infra-red light. The
temperature that allows these energy sources and sinks to balance is
called the radiative equilibrium temperature.
- The Greenhouse Effect allows certain planets to warm
up above their respective radiative equilibrium temperatures. The effect
is due to the presence in the atmosphere of Greenhouse gases, such as CO2,
H2O, NO, etc.
Global Warming Potential of
Greenhouse Gases
and the Energy (Im)Balance of Our Current Atmosphere
Driving
Questions:
- What's processes lead to temperature change?
- What gases comprise the atmosphere and how do they
influence the temperature?
- What are the global warming potentials for greenhouse
gases?
1. Natural Climate Change
We believe that the temperature of the earth
has varied wildly over the evolution of the earth. Figure 1 shows an estimate
of temperature changes as complied by Scotese. So how can it be that the
climate has changed so over the ages and what processes could lead to these
changes?
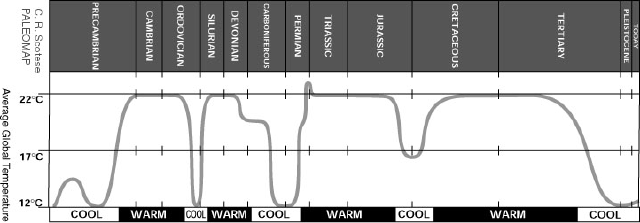
Figure 1. Estimated changes in global temperature
The processes for changing climate naturally
include:
- Plate tectonics
- Volcanic eruptions
- Solar Variations, and
- Orbital Variations
Plate Tectonics
The movement of the continents has obviously
influenced the climate at specific locations (Figure 2), but could also
influence the global temperature by redistributing the collection of solar
radiation and/or providing land masses on which continental glaciers could
form.
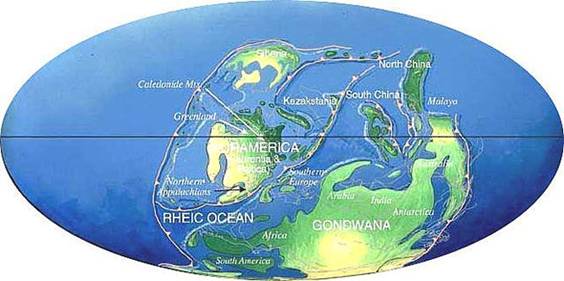
Figure 2. Location of continents during the Devonian
period from Scotese
Volcanic Eruptions
The amount and location of material added to
the atmosphere by volcanoes probably has significantly influenced climate over
the ages. Volcanoes emit some greenhouse gases like CO2 and H2O,
but also emit SO2 that can get trapped in the upper parts of the
atmosphere where it will react to form sulfates, a small particle. These
particles can reflect incoming radiation to lower the surface temperature.
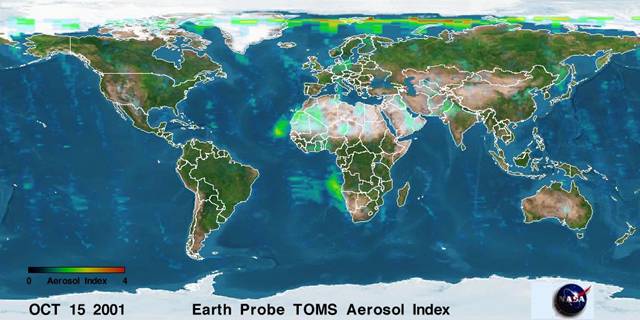
Figure
3. NOAA monitors the amount or particles (aerosols) in the atmosphere. Note
aerosols over northern and southern
Solar Variability
Variations in sunspot activity result in
changes on the order of 0.1% to 0.2 over 11 year cycle. Numerical climate
models predict that a change in solar output of only 0.5% per century could
alter the Earth's climate.
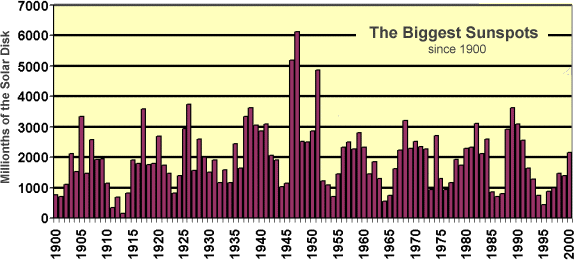
Figure
4. The changing amount of the solar surface covered by the biggest sunspots,
Earth would be 169 millionths on the chart. (Courtesy Rich J Niciejewski, U.
Orbital Variability
The Earth's orbit changes over time in ways
that could influence the amount of energy received at the surface. These
include changes in eccentricity, precession of the equinox, and changes in the
Earth's tilt (obliquity).
Eccentricity
The
eccentricity of the Earth's orbit changes with a period of 100,000 years. At
the moment the Earth's orbit is fairly circular but in 50,000 years it will be
more eccentric with the difference between aphelion (farthest) and perihelion
(nearest) points in the orbit will become larger.
Precession of the Equinox
Now the Earth is closest the sun in January
and farthest in July. The combination of changing eccentricity and precession
of the equinox leads to changing available solar radiation.
Obliquity
Finally, the Earth wobbles on its axis of
rotation changing the tilt of the Earth (and hence its seasonality) over a
41,000 year period. The tilt is now 23.5¼ but changes between 22.5¼ and 24.5¼.
![]()
2. Radiative Forcing
The temperature of the Earth's surface and
atmosphere are dictated by a balance between incoming energy and outgoing
energy. Temperature rises when more energy is received than lost. The Earth's
surface, for example, absorbs radiation from the Sun. This energy is then
redistributed by the atmospheric and oceanic circulations and radiated back to
space at longer (infrared) wavelengths. For the annual mean and for the Earth
as a whole, the incoming solar radiation energy is balanced approximately by
the outgoing terrestrial radiation. Any factor that alters the radiation
received from the Sun or lost to space, or that alters the redistribution of
energy within the atmosphere and between the atmosphere, land, and ocean, can
affect climate. A change in the net radiative energy available to the global
Earth-atmosphere system is termed a radiative forcing. Positive
radiative forcing tends to warm the Earth’s surface and lower atmosphere.
Negative radiative forcing tends to cool them.
Increases in the concentrations of greenhouse
gases will reduce the efficiency with which the Earth’s surface radiates to
space. More of the outgoing terrestrial radiation from the surface is absorbed
by the atmosphere and re-emitted at higher altitudes and lower temperatures.
This results in a positive radiative forcing that tends to warm the lower
atmosphere and surface. Because less heat escapes to space, this is the
enhanced greenhouse effect – an enhancement of an effect that has
operated in the Earth’s atmosphere for billions of years due to the presence of
naturally occurring greenhouse gases: water vapor, carbon dioxide, ozone,
methane and nitrous oxide. The amount of radiative forcing depends on the size
of the increase in concentration of each greenhouse gas, the radiative properties
of the gases involved (indicated by their global warming potential), and
the concentrations of other greenhouse gases already present in the atmosphere.
Further, many greenhouse gases reside in the atmosphere for centuries after
being emitted, thereby introducing a long-term commitment to positive radiative
forcing.
Anthropogenic aerosols (microscopic airborne
particles or droplets) in the troposphere, such as those derived from fossil
fuel and biomass burning can reflect solar radiation, which leads to a cooling
tendency in the climate system. Because it can absorb solar radiation, black
carbon (soot) aerosol tends to warm the climate system. In addition, changes in
aerosol concentrations can alter cloud amount and cloud reflectivity through
their effect on cloud properties and lifetimes. In most cases, tropospheric
aerosols tend to produce a negative radiative forcing and a cooler climate.
They have a much shorter lifetime (days to weeks) than most greenhouse gases
(decades to centuries), and, as a result, their concentrations respond much
more quickly to changes in emissions. Volcanic activity can inject large
amounts of sulfur-containing gases (primarily sulfur dioxide) into the
stratosphere, which are transformed into sulfate aerosols. Individual eruptions
can produce a large, but transitory, negative radiative forcing, tending to
cool the Earth’s surface and lower atmosphere over periods of a few years.
When radiative forcing changes, the climate
system responds on various time-scales. The longest of these are due to the
large heat capacity of the
Figure
5. Global average
flow of shortwave (solar) radiation through the Earth's atmosphere
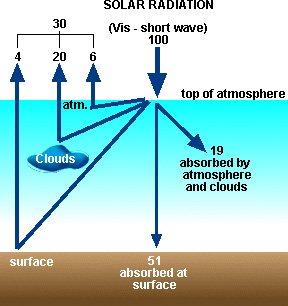
Shortwave Radiation Budget
Solar radiation entering the Earth's
atmosphere (called "shortwave" radiation) can be reflected off
clouds, the surface, and air molecules and dust. On a global average this
accounts for about 30% of incoming radiation (see Figure 5). This percentage is
quantified as the albedo of the system.
Albedo
= percentage of incoming radiation that is reflected back into space = 30% for
Earth
Another 19% on average is absorbed by the
atmosphere, mainly by ozone in the Earth's stratosphere. The remaining 51% is
absorbed by the Earth's surface.
Over a long term average, the Earth and its
atmosphere must radiate as much energy out to space as it receives from the
sun, but over the course of a year or a day or as one moves geographically it
is likely that such a balance will not be present. At night and in the winter,
for example, there is less solar radiation producing an energy deficit and leading
to lower temperatures at those times in general.
In order to understand the whole energy
balance we must also consider the other means for exchanging energy between the
Earth's surface, atmosphere and space.
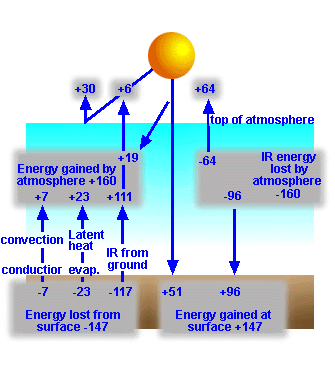
Figure
6: Energy
exchange between the Earth's surface and its atmosphere.
Gains
|
Losses
|
||
|
|
|||
|
51 |
Visible from Sun |
7 |
Conduction, Convection |
|
96 |
IR from atmosphere |
23 |
Evaporation |
|
|
|
117 |
IR radiation |
|
|
|||
|
147 |
net |
147 |
net |
Longwave Radiation Budget
As was learned earlier all objects emit radiation
in an amount and at a wavelength dictated by the object's temperature. The 51%
of shortwave radiation absorbed by the Earth's surface (Figure 5) heats the
surface. But as the surface heats it emits radiation in the infrared back into
the atmosphere.
Figure 6 shows the annual global average
exchange of energy between the Earth's surface and the atmosphere. Note the 51%
of original solar radiation is absorbed, but 117% of the original solar input
is emitted to the atmosphere, how can this be?
The answer makes sense when we consider that
the surface of a planet receives a great deal of energy from its own
atmosphere. Thus the effect of the atmosphere is to warm the surface over the
temperature above that resulting from the Sun's energy.
The atmosphere warms the Earth by
"trapping" radiation, allowing the surface to warm to 300°K. At that
temperature, the black body surface radiation is large enough to ensure that an
equilibrium condition pertains. The atmosphere traps radiation through the action
of certain gases, called Greenhouse Gases. These gases (e.g., CO2, H2O,
NO, CFCs, CO) are very good at absorbing and re-emitting infrared radiation.
They intercept the IR radiation from the ground and reflect some of the energy
back to the ground, warming it up more than would occur otherwise.
3. Global Warming Potential
The Global Warming Potential (GWP) of a
greenhouse gas is the ratio of global warming, or radiative forcing – both
direct and indirect – from one unit mass of a greenhouse gas to that of one
unit mass of carbon dioxide over a period of time. Hence this is a measure of
the potential for global warming per unit mass relative to carbon
dioxide.
Global Warming Potentials are presented in
Table 1 for an expanded set of gases. GWPs are a measure of the relative
radiative effect of a given substance compared to CO2, integrated
over a chosen time horizon. New categories of gases in Table 1 include
fluorinated organic molecules, many of which are ethers that are proposed as
halocarbon substitutes. Some of the GWPs have larger uncertainties than that of
others, particularly for those gases where detailed laboratory data on
lifetimes are not yet available. The direct GWPs have been calculated relative
to CO2 using an improved calculation of the CO2 radiative
forcing, the SAR response function for a CO2 pulse, and new values
for the radiative forcing and lifetimes for a number of halocarbons. Indirect
GWPs, resulting from indirect radiative forcing effects, are also estimated for
some new gases, including carbon monoxide. The direct GWPs for those species
whose lifetimes are well characterized are estimated to be accurate within
±35%, but the indirect GWPs are less certain.
|
Table
1. Direct
Global Warming Potentials (GWPs) relative to carbon dioxide (for gases for
which the lifetimes have been adequately characterized). GWPs are an index
for estimating relative global warming contribution due to atmospheric
emission of a kg of a particular greenhouse gas compared to emission of a kg
of carbon dioxide. GWPs calculated for different time horizons show the
effects of atmospheric lifetimes of the different gases. |
|||||
|
|
|
Lifetime |
Global Warming Potential |
||
|
|
|
(years) |
(Time Horizon in Years) |
||
|
GAS |
|
|
20 yrs |
100 yrs |
500 yrs |
|
Carbon
Dioxide |
CO2 |
|
1 |
1 |
1 |
|
Methane |
CH4 |
12.0 |
62 |
23 |
7 |
|
Nitrous
Oxide |
N2O |
114 |
275 |
296 |
156 |
|
Chlorofluorocarbons |
|
|
|
|
|
|
CFC-11 |
|
55 |
4500 |
3400 |
1400 |
|
CFC-12 |
|
116 |
7100 |
7100 |
4100 |
|
CFC-115 |
|
550 |
5500 |
7000 |
8500 |
|
Hydrofluorocarbons |
|
|
|
|
|
|
HFC-23 |
CHF3 |
260 |
9400 |
12000 |
10000 |
|
HFC-32 |
CH2F2 |
5 |
1800 |
550 |
170 |
|
HFC-41 |
CH3F |
2.6 |
330 |
97 |
30 |
|
HFC-125 |
CHF2CF3 |
29 |
5900 |
3400 |
1100 |
|
HFC-134 |
CHF2CHF2 |
9.6 |
3200 |
1100 |
330 |
|
HFC-134a |
CH2FCF3 |
13.8 |
3300 |
1300 |
400 |
|
HFC-143 |
CHF2CH2F |
3.4 |
1100 |
330 |
100 |
|
HFC-143a |
CF3CH3 |
52 |
5500 |
4300 |
1600 |
|
HFC-152 |
CH2FCH2F |
0.5 |
140 |
43 |
13 |
|
HFC-152a |
CH3CHF2 |
1.4 |
410 |
120 |
37 |
|
HFC-161 |
CH3CH2F |
0.3 |
40 |
12 |
4 |
|
HFC-227ea |
CF3CHFCF3 |
33 |
5600 |
3500 |
1100 |
|
HFC-236cb |
CH2FCF2CF3 |
13.2 |
3300 |
1300 |
390 |
|
HFC-236ea |
CHF2CHFCF3 |
10 |
3600 |
1200 |
390 |
|
HFC-236fa |
CF3CH2CF3 |
220 |
7500 |
9400 |
7100 |
|
HFC-245ca |
CH2FCF2CHF2 |
5.9 |
2100 |
640 |
200 |
|
HFC-245fa |
CHF2CH2CF3 |
7.2 |
3000 |
950 |
300 |
|
HFC-365mfc |
CF3CH2CF2CH3 |
9.9 |
2600 |
890 |
280 |
|
HFC-43-10mee |
CF3CHFCHFCF2CF3 |
15 |
3700 |
1500 |
470 |
|
Fully
fluorinated species |
|
|
|
|
|
|
SF6 |
|
3200 |
15100 |
22200 |
32400 |
|
CF4 |
|
50000 |
3900 |
5700 |
8900 |
|
C2F6 |
|
10000 |
8000 |
11900 |
18000 |
|
C3F8 |
|
2600 |
5900 |
8600 |
12400 |
|
C4F10 |
|
2600 |
5900 |
8600 |
12400 |
|
c-C4F8 |
|
3200 |
6800 |
10000 |
14500 |
|
C5F12 |
|
4100 |
6000 |
8900 |
13200 |
|
C6F14 |
|
3200 |
6100 |
9000 |
13200 |
|
Ethers
and Halogenated Ethers |
|
|
|
|
|
|
CH3OCH3 |
|
0.015 |
1 |
1 |
<<1 |
|
HFE-125 |
CF3OCHF2 |
150 |
12900 |
14900 |
9200 |
|
HFE-134 |
CHF2OCHF2 |
26.2 |
10500 |
6100 |
2000 |
|
HFE-143a |
CH3OCF3 |
4.4 |
2500 |
750 |
230 |
|
HCFE-235da2 |
CF3CHClOCHF2 |
2.6 |
1100 |
340 |
110 |
|
HFE-245fa2 |
CF3CH2OCHF2 |
4.4 |
1900 |
570 |
180 |
|
HFE-254cb2 |
CHF2CF2OCH3 |
0.22 |
99 |
30 |
9 |
|
HFE-7100 |
C4F9OCH3 |
5 |
1300 |
390 |
120 |
|
HFE-7200 |
C4F9OC2H5 |
0.77 |
190 |
55 |
17 |
|
H-Galden 1040x |
CHF2OCF2OC2F4OCHF2 |
6.3 |
5900 |
1800 |
560 |
|
HG-10 |
CHF2OCF2OCHF2 |
12.1 |
7500 |
2700 |
850 |
|
HG-01 |
CHF2OCF2CF2OCHF2 |
6.2 |
4700 |
1500 |
450 |
Summary
- Greenhouse gases selective absorb infrared radiation,
thus trapping energy in the atmosphere.
- The atmosphere radiates energy to the surface at an average
rate greater than the rate of incoming solar radiation.
- Each greenhouse gas is characterized by its
atmospheric lifetime and global warming potential.
Suggested Readings
- Ahrens, C. Donald, "Meteorology Today", 6th
ed., Brooks/Cole Publishing Co., 1999.
- IPCC Technical Summary of the
Working Group I Report (pdf), 2001.