Unit 8. Climate: Atmosphere and Oceans
Earth’s atmosphere is key to the origin and evolution of life on our planet, which
distinguishes our planet from others in the solar system. The original atmosphere was formed in a very
hot and turbulent early Earth, from outgassing of trapped volatiles. Several processes cooled our planet’s surface,
allowing the formation of oceans that, in turn, permitted the evolution of O-producing
life. The diversity of life is intimately
coupled with atmospheric and ocean circulation, which explain today’s regional climate
patterns and biomes distribution. Today’s
atmosphere is being altered by the rapid increase of greenhouse gases, leading
to new challenges for modern life. These
topics are explored in this unit.
How
did Earth’s atmosphere and oceans form?
Following accretion into a large
planet-sized object during the early years of the solar system, Earth’s first
major atmosphere was formed by the release of gases trapped in the restless
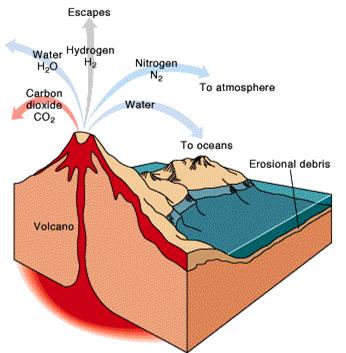
interior, a process that still goes on today in volcanoes (Figure 8.1). These
early years are marked by swirling oceans of hot magma that no longer exists
today on any planet in our solar system.
Extreme volcanism in Earth’s early history occurred in response to this
energetic motion of then-molten mantle material. As planetary material
violently overturned, volatile gases from the interior, especially Carbon
dioxide (CO2), Carbon monoxide (CO), Hydrogen (H2),
Nitrogen (N2) and water vapor (H2O), were released, and
accumulated in a gaseous surface layer that was trapped by gravitational
forces. Radiation from the nearby Sun swept lighter gases as H and He away,
leaving only heavier molecules in this early atmosphere. Chemical reactions in the hot surface layer
formed other simple atmospheric compounds, such as methane (CH4) and
ammonia (NH3). While far less abundant, the latter compounds are
highlighted as they are key components of amino acids, which are the
fundamental building blocks of life’s proteins.
Note also that Oxygen (O2), key to the survival of many forms
of modern life, was not present in the early atmosphere.
|
|
Figure 8.1Volcanic outgassing. |
Water vapor is the dominant form
of gas released in outgassing, so much that Earth’s early atmosphere became
saturated with water, leading to an era of continuous rain on the planet. These
rains contributed to cooling of the Earth’s surface and the formation of the
first oceans, which today cover two thirds of the surface. Another contributing process to the surface
heat budget was the permanent removal of CO2 from the atmosphere by
the formation of carbonate rocks (consisting mostly of the mineral calcite,
CaCO3), which reduced the atmosphere’s greenhouse potential and
lowered the surface temperature.
The continuous rains and
atmospheric CO2 removal cooled our planet and created a surface
where water could eventually be present in all three phases: vapor, liquid and
solid. Ocean formation made Earth
habitable for life at about 4000 million years ago, leading to today’s highly
complex organisms. The origin of life
remains heavily debated, but the changes in organism over billions of years
would not have been possible without surface conditions that persist today.
Life began to have a major
impact on the environment once simple photosynthetic organisms, like blue-green
algae, evolved in the oceans. These organisms fed off solar energy and CO2,
and produced oxygen (O2) as a waste product. For hundreds of
millions of years, the oxygen that the algae produced was dissolved in ocean
water and reacted with iron rich sediment in a process called oxidation, but O
did not build up as a gas in the atmosphere. It was not until approximately 2
billion years ago that the reservoirs of oxidizable
material became saturated and free oxygen built up to significant amounts in
the atmosphere. Today, the outgassing process continues as volcanism, releasing
mainly H2O, CO2, SO2 and lesser amounts of
gases such as N2, albeit in much less dramatic form, while the O
budget is maintained by surface life.
Why
does Earth have water in all three physical states?
The distance at which each
planet orbits the Sun influences the surface temperature. For example, Venus
being closer to the Sun, receives a higher amount of solar radiation than
Earth, thus the surface temperature of Venus is higher than Earth. Mars is therefore
cooler. However, in addition to a
planet’s distance from the Sun, the atmospheric composition of each planet has
major impacts on the resulting planetary surface temperature.
Venus has a surface pressures
that is much too high and temperatures that are too
warm for liquid water to develop, because of a high concentration of CO2 in its
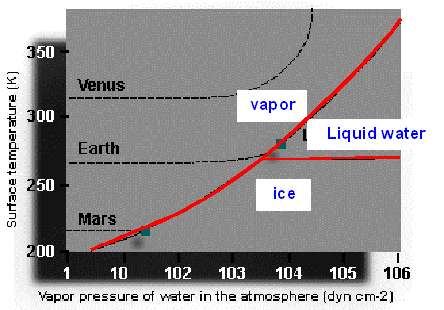
atmosphere (Figure 8.3). Any water on Venus will be in the form of water vapor,
because a surface temperature of >450o C and a high atmospheric
surface pressure are too high for liquid water to form. Vapor saturation of H2O on Venus
is never reached, so the atmosphere continues to accumulate H2O. Today’s atmosphere of Venus is more than 98%
CO2, with no oxygen or liquid water, which led to the concepts
of the runaway greenhouse. The thick
atmosphere of CO2 and water vapor trap incoming solar radiation and
make the surface of the planet extremely hot, without a cooling process to
provide a negative feedback.
|
|
Figure 8.3 H2O phase diagram and occurrences on the planets Venus, Earth and Mars |
Modern Earth is at the right
distance from the Sun (150 x 106 km) and with a suitable atmosphere,
so that H2O is able to form in all three phases: gaseous (vapor),
liquid (water) and solid (ice). After the differentiation and outgassing period
in Earth’s early history, the water vapor that accumulated in the atmosphere
changed to torrential rains that lasted millions of years, leading to the first
oceans. The formation of oceans was likely complemented by the impact of icy
comets that brought water to the surface.
Once liquid water became available at the surface, the atmospheric
composition of Earth changed further, as atmospheric species that were soluble
in water dissolved into the rain and oceans.
Because CO2 is soluble in water it was slowly removed from
the atmosphere leaving the relatively scarce but non-reactive nitrogen (N2)
to build up in the atmosphere, to today’s value of 78%. This is one of the key
differences between Earth’s atmosphere and those of Venus and Mars that are
dominated by CO2. Earth’s average surface temperature is today ~15o
C and the surface pressure is approximately 1,000 mb,
which allows the formation of H2O in liquid form, gaseous water
vapor in the atmosphere, and ice at the colder poles (Figure 8.3).
In comparison to Earth, Mars is
too cold for liquid water to form, as it is farther from the Sun (at 228 x 106
km) than Earth. Therefore, Mars receives
less solar radiation than Earth and Venus. The relatively low Martian surface
temperatures of –60o C and low surface pressure at 6 mb results in water vapor forming ice. The thin Martian
atmosphere is currently made of 96% CO2, 2.5% oxygen and has only
trace amounts of water, but in its earlier history likely had flowing water
that formed the famous Martian channels.
Why is Earth’s
atmosphere dynamic?
Earth’s atmosphere has evolved through
volcanic outgassing and from the evolution of oxygen producing organisms
approximately 3.5 billion years ago, which changed the composition of the
atmosphere from an oxygen-less planet to one that could support complex life. Today’s
dominant changes in the atmosphere are occurring due to human activities, such
as fossil fuel combustion and land use change, which are altering the global
climate through changes in greenhouse gas concentrations, reflectivity of the
atmosphere, and alteration of precipitation patterns. Currently, the atmosphere
consists of approximately 78% nitrogen, 21% oxygen, 0.9% argon, close to 0.04%
carbon dioxide, and a plethora of other elements, compounds, and particulate
aerosols.
Earth’s atmosphere is held to
the planet by gravity and from thermal movement of the air molecules. Radiative energy from the Sun, the rotation of the Earth
and the distribution of land and water, are the drivers for Earth’s physically
dynamic atmosphere. Incoming solar radiation is at the highest angle and
therefore strongest at the equator, and highly oblique and therefore weakest at
the Earth’s polar regions. This causes uneven heating
of atmospheric air parcels, where warm air at the equator rises and cooler air
at the poles descends (a north-south gradient). However, the Earth’s rotation also
pushes the air parcels around the Earth, but in an east-west manner. The temperature gradients and the rotation of
the Earth together create convective cells of atmospheric circulation on the
planet. These processes produce prevailing winds and latitudinal belts of low
and high air pressure around the globe.
|
|
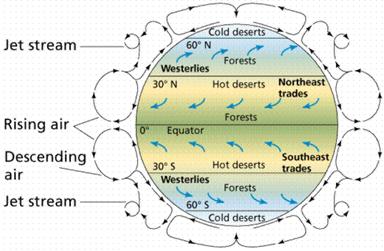
Figure
8.2 Global atmospheric circulation.
http://www.emc.maricopa.edu‑/faculty/farabee/BIOBK/deserts.gif |
Temperature is a measure of the
thermal energy or the movement of molecules and refers to the heat of an object:
temperature equals the mean atomic speed squared: T = v2. Air
parcels with higher energy, meaning warmer, are less dense so they tend to
rise. Pressure is a measurement of the force per unit area. Atmospheric
pressure is the weight of the atmosphere per unit area, above the point of
measurement. Therefore, as you move away from the Earth’s surface toward space,
the atmospheric pressure decreases, i.e. the weight of the atmosphere is less
because there is less air mass above you. In addition to temperature and
pressure, the amount of water vapor that is present in the atmosphere plays a role
in air movement, because it affects the temperature of the atmosphere.
Climate and weather patterns are
strongly related to the position of the convective atmospheric cells and belts
of atmospheric pressure around the planet. For example, many of the world’s hot
deserts, regions of very low precipitation, reside near 30o N and 30o
S latitude (Figure 8.2). This is because these locations correspond to areas of
high pressure where cool and dry air from the atmosphere descends and picks up
moisture from the Earth’s surface. As the air warms and picks up moisture, it
moves toward the low pressure-belts of the equator and near 50-60o N
and S latitude, and in the process releases much of the moisture as rainfall.
As the rising air parcel releases the rain, it cools and cycles
back toward higher latitudes and repeats the loop. This process of
releasing moisture over the equator determines why much of the equatorial
region is tropical with high amounts of rainfall. The circulation of the
Earth’s atmosphere, and the high and low pressure
belts transfer heat and precipitation around the world. The wide spectrum of
moisture and temperature gradients that result from this dynamic atmosphere
adds to the great diversity of organisms that are found on Earth.
What
is the function of the great ocean conveyer belt?
The Earth’s oceans cover approximately 71% of the globe and contain 97% of the water on the planet. Oceans also move absorbed solar radiation in the form of heat around the world; thus, oceans are extremely important for climate regulation on Earth. For example, the oceans take up over half of the solar radiation absorbed on Earth, with ocean currents then redistributing this energy. A key attribute of ocean water is its ability to hold dissolved ions in solution. In addition to other solutes, seawater has approximately 3.47% dissolved salt, which differs slightly by ocean region. As the salinity of water increases, the density of the liquid also increases, which has profound effects on ocean circulation and nutrient transfer rates.
Unlike the atmosphere, which is
characterized by turbulent weather systems, the ocean is a fairly stable
because it is heated from above, in contrast to the atmosphere, which is heated
from below. The relatively warm surface waters, therefore, tend to remain
floating on top of the colder and denser deeper waters. This tendency generally
leads to a stable situation that inhibits turbulence. If the deep ocean were
never to move, however, conditions would quickly become anoxic or void or
oxygen. Because ocean waters are not usually anoxic there must be another
mechanism for deep-ocean mixing.
When surface ocean water absorbs
radiation, it heats up and eventually evaporates, leaving behind dissolved
salts. This evaporative process leaves the remaining water with a high solute
concentration and thus more dense, than the water below it. The dense, saline
water on the surface tends to sink to the ocean floor, causing ocean mixing and
deep ocean currents. In addition to the evaporative driver for changes in ocean
water salinity, sea ice formation also contributes to saline polar waters. As
sea ice forms, solutes are not incorporated into the ice crystals, thus the
solutes are left behind and increase the salinity of the surrounding water.
The sinking action of saline
water produced by a combination of temperature and salinity is called
thermohaline circulation, and drives large-scale ocean circulation over century
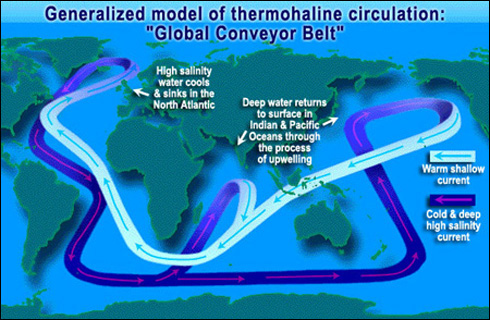
long time scales. Large-scale circulation of the oceans is deemed the great
ocean conveyer belt and involves a deep current, which carries more than 30
times the volume of all the rivers of the world combined (Figure 8.4). The
conveyor belt is an immense ocean current that transfers heat from the warm
tropics to the cold, polar-regions. For instance, cold, salty, North Atlantic
waters sink deep into the ocean and travel south around Africa. The water
either rises to the surface near the tropical waters of India, or the cold
saline water continues to move along Antarctica and travels up the Eastern side
of Australia to the Southern coast of Alaska where the current rises toward the
surface along the west coast of North America. This warmer shallower current
then moves through the tropical equatorial waters of
In addition to the transfer of
heat around the globe, the great ocean conveyor belt moves nutrient-rich water
around the ocean. The ocean conveyor belt also moderates the climate in regions
such as
|
|
|
Figure 8.4 The great ocean conveyor belt
that circulates warm and cold ocean water around the globe. http://science.hq.nasa.gov/oceans/images/CONVEYOR.jpg |
Why
is the global climate changing?
In a nutshell, humans are
altering the radiative balance of the Earth. The
balance between incoming energy and outgoing energy dictates the temperature of
the Earth. Temperatures increase when more energy is received than lost. The
Earth's surface absorbs shortwave radiation from the Sun. This energy is then
redistributed by atmospheric and oceanic circulation and radiated back to space
at longer, infrared wavelengths. On average for the Earth as a whole, the
incoming solar radiation energy is balanced approximately by the outgoing
terrestrial radiation. Global climate can be affected by any factor that alters
the radiation received from the Sun or lost to space, or that alters the
redistribution of energy within the atmosphere and between the atmosphere,
land, and ocean. A change in the net radiative energy
available to the global Earth-atmosphere system is termed radiative
forcing. Positive radiative forcing tends to warm the
Earth’s surface and lower atmosphere, whereas negative radiative
forcing tends to cool them.
Some amount of positive radiative forcing is essential for life to exist on Earth.
Without the natural amount of radiative forcing or
the natural greenhouse effect, the temperature on Earth would be too cold to
support life. This natural greenhouse effect warms Earth to conditions that are
conducive to support life, and should be distinguished from the anthropogenic
greenhouse effect that is currently causing so much concern. Human activities
are currently changing the amount of greenhouse gases in the atmosphere,
thereby increasing positive radiative forcing and
warming the planet.
Since the industrial revolution,
humans have increased the amount of greenhouse gases in the atmosphere due to
the burning of ancient carbon stored in the Earth, in the form of coal, oil,
and natural gas, which releases CO2, an important greenhouse gas. In
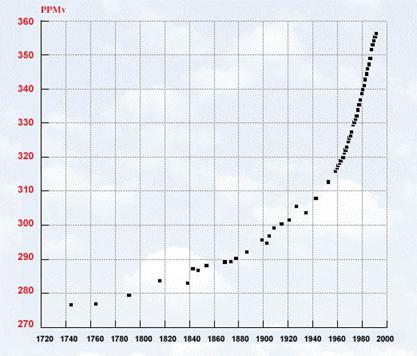
the last one hundred years, humans have increased the CO2
concentration in the atmosphere by more than 100 parts per million (ppm; Figure 8.5). In the next 100 years scientists estimate
that concentrations will increase by another 200 ppm
or more to greater than 600 ppm. The current concentration
of CO2 in the atmosphere at 388 ppm is
greater than we have seen in the last 650,000 years! Furthermore, in addition
to increases in CO2 from fossil fuel combustion that is increasing
greenhouse warming, there are many other greenhouse gases on the rise, such as
methane and nitrous oxide, which are also contributing to global warming.
|
|
Figure 8.5CO2 concentration over time http://images.wri.org/chart_scan_cni_f04b.gif |
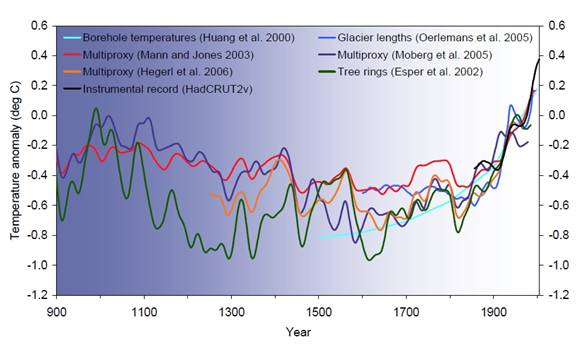
An Increase in the concentration of greenhouse gases reduces the efficiency with which the Earth’s surface radiates energy back to space. As the amount of greenhouse gases increases, more of the outgoing terrestrial radiation from the surface is absorbed by the atmosphere and re-emitted at higher altitudes and lower temperatures. This process results in positive radiative forcing that tends to warm the lower atmosphere and Earth’s surface (Figure 8.6). Because less heat escapes to space, this is the enhanced greenhouse effect – an enhancement of an effect that has operated in the Earth’s atmosphere for billions of years due to the presence of naturally occurring greenhouse gases: water vapor, carbon dioxide, ozone, methane and nitrous oxide.
|
|
|
Figure
8.6 Temperature record for the past 1000 years |
The amount of positive radiative forcing depends on the size of the increase in
concentration of each greenhouse gas, the radiative
properties of the gases involved (indicated by their global warming potential),
and the concentrations of other greenhouse gases already present in the
atmosphere. Further, many greenhouse gases reside in the atmosphere for
centuries after being emitted, thereby resulting in a long-term commitment to
positive radiative forcing. For example, CO2
has an atmospheric residence time of 100 years. Therefore, the CO2
that you emitted to the atmosphere today will still be warming the planet when
your great grandchildren are born.
How
is the composition of the troposphere changing?
The troposphere is 10-12 km
thick and is the layer of the atmosphere that is closest to the Earth’s
surface. The troposphere contains 90% of the Earth’s atmosphere and is the
layer in which weather occurs. In addition, the troposphere is the layer in
which human beings and other living organisms inhabit. Because humans live in
the troposphere, this layer of atmosphere is also the layer that receives the
majority of atmospheric wastes such as CO2, nitrogen oxides, and
sulfur oxides. The temperature of the troposphere decreases as one moves away from
the Earth’s surface towards the stratosphere, which is the second layer of the
Earth’s atmosphere. The boundary between the troposphere and the stratosphere
is deemed the tropopause, which acts like a lid to
trap moisture in the troposphere.
Human activities have
substantially altered the composition of the troposphere in the last 200 years.
The burning of fossil fuels is one of the main drivers of tropospheric
changes. Fossil fuel emissions have increased the concentration of CO2,
CO, methane, nitrogen oxides, sulfur oxides, and chlorofluorocarbos
(CFCs) in the atmosphere. Many of these gases are greenhouse gases meaning that
they selectively absorb radiation at certain wavelengths and trap heat from
escaping into space, altering the radiative equilibrium
of the planet. In addition to the increase in greenhouse gases, human
activities have significantly increased the amount of dust, smoke, and
particulate aerosols in the troposphere, which alters the albedo
or reflectivity of the atmosphere and plays a role in cloud formation. Changes
in the reflectivity of the atmosphere and the amount of cloud cover can also
play major roles in regional and global changes in temperature.
The massive increase in atmospheric
pollution resulting from human activities has major consequences for the health
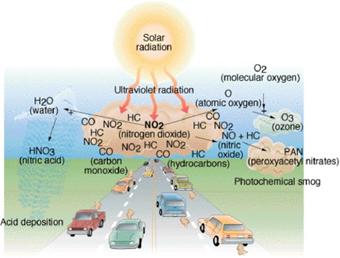
of ecosystems and people alike. For example, noxious tropospheric
smog and ozone develop over urban areas from the combination of nitrogen oxides
interacting with sunlight (Figure 8.7). Photochemical smog, tropospheric
ozone, and particulate aerosols can cause major health consequences such as
asthma, cancer, and respiratory and heart problems in humans and animals. In
addition, atmospheric pollution can cause acid rain and can damage
photosynthetic systems in plants and decrease agricultural yield. In addition,
because of the dynamic nature of the Earth’s atmosphere, noxious air pollution
formed in urban and industrialized areas can travel to many other regions of the
globe, which can cause far-reaching climatic and health consequences for people
and ecosystems.
Another major driver for changes
in the composition of the troposphere is land use change. Land use changes like
deforestation and conversion of forestlands to agricultural lands impact
surface albedo, as well as the amount of dust,
aerosols, and particulates that are present in the atmosphere. Atmospheric dust
and particulates can increase due to erosion and desiccation of soil from land
use change. Removal of forest vegetation, especially tropical forest
vegetation, also has a major impact on the amount of water vapor that is
present the in the atmosphere. Vegetation removal reduces evapotranspiration
from the Earth’s surface, creating warmer, dryer atmospheric conditions, which
has consequences for local, regional, and global weather patterns. For example,
in the Amazon basin, more than half of the rainfall occurring within the basin
is a result of evapotranspirative recycling from
within the basin itself. Experts predict that major deforestation within the
basin could cause profound changes in the regional weather patterns and
potentially prevent rainforest regeneration.
|
|
Figure 8.7 Tropospheric photochemistry.http://www.uwm.edu/Course/416-120/Chapter02/fg03_011.jpg |
Last updated: 8/30/2006 10:32 AM