Unit 10. Global Biogeochemistry: Transformations and Cycles
How
do we measure biogeochemical cycles?
Biogeochemistry is the study of
how the geological, chemical, and biotic systems on Earth interact and
influence each other. The study of biogeochemistry
is often focused on how particularly important elements such as carbon or
nitrogen are linked and affected by geological, chemical, and biological
systems. This linkage affects the cycling of all the important elements. In
addition, the biogeochemical cycles of individual elements are strongly
connected and interact with each other. Therefore, alterations in one cycle
such as the hydrologic cycle, can forcefully impact the functioning of other
element cycles. It is important that we understand Earth’s biogeochemical
cycles because humans are altering their balance. Humans currently control and
consume more than 40% of the primary production (carbon and nitrogen cycles)
generated on land and in the oceans, and since the human population is increasing
at an exponential rate this rate of consumption is set to increase over time.
Humans have already substantially altered the Earth’s biogeochemical cycles by
changing steady-state systems that have been in balance for thousands or
millions of years. For example, humans have strongly altered the balance of the
carbon (C) cycle by burning fossil fuels and increasing the concentration of C
in the atmosphere. Thus, it is important that we understand how our actions
impact these cycles in order to predict the consequences of our actions, and to
protect the sustainable balance of the planet for ourselves and for the other
organisms that depend on these cycles.
Biogeochemists use
several different “tools” to understand changes in biogeochemical systems. They
calculate flux rates, or the movement of a material through a given area over a
specific amount of time. In addition to flux measurements, we need to be able
to measure the amount of material that is in the reservoir or pool that holds
the material that we are interested in measuring. There are four main
reservoirs or pools that we need to understand to measure Earth’s
biogeochemical cycles: the atmosphere, land, ocean, and rocks. The “cycling” is
simply the movement of elements between these pools – for example, primary
production moves C from the atmosphere pool to the land pool, and respiration
of organic matter moves the C back to the atmosphere pool (see Unit 4). Cycles
involving these four pools interact with one another and feedback within and
between the reservoirs. To understand cycles, we must also be able to calculate
the rate of movement between pools, and determine what factors drive or control
the cycling between pools. To do this, it is useful to use the principles of
mass balance.
Mass balance equations are often
used to describe the state of a system because they can be used to measure both
the flux and the change in pool size in a given system. These mass balance
equations operate in the same way regardless of the scale or size of the system
of interest. In general, how much a system changes, or the net change in a
system due to a perturbation, is dependent upon the amount of input plus the
output, plus the internal change.
Net
Change = Input + Output + Internal Change
Let’s examine a simple system using a mass balance equation. Say you have $500 in your savings account. This is considered your pool or reservoir. Your bank gives you 10% interest on the money in your account each payday (month). Therefore your internal change is 10% of $500 or $50. On payday, you deposit $100 into your account; this is your input. However, your monthly rent is due the next day, so you take out $200 to pay it; this is your output. Therefore to calculate your net change, you simply add up all the components: Net Change = Input ($100) + Output (-$200) + Internal Change ($50) = $ -100 + 50 = $-50. Therefore your net change is minus $50 (Figure 10.1), which means that your initial pool size of $500 will decrease and now you will only have $500 – 50 = $450 in your account. Notice that in this case there is a relatively small pool size ($500) and a relatively large net change or flux rate (-$50 per month), so the system will fall out of its initial balance rapidly. In fact, your reservoir of money will be gone in a matter of months! Large pool sizes on the other hand are often difficult to disturb; for example, if you have $500,000 in the bank, and still only had an internal change of $50 and the same rent and income, your net change would be small relative to the pool size and your reservoir would last a long time.
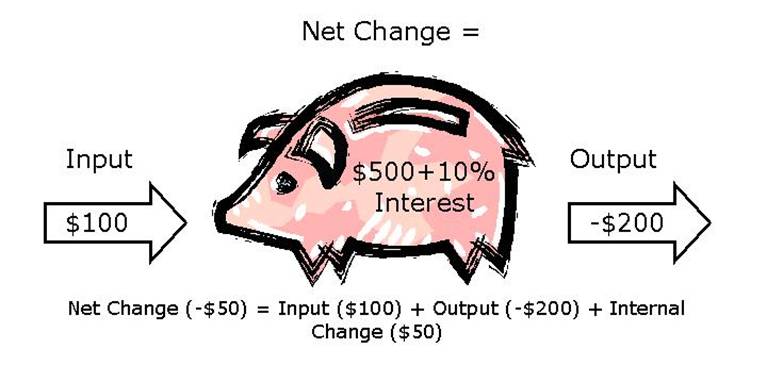
Figure 10.1
Mass balance example
This bank account example can be easily translated to the world of science by considering the mass balance of tropical forests on Earth. In this equation there is a tremendous output (loss) of forest caused by human cutting and burning, humans are not adding new forest (zero input), and the internal change or natural growth of new forest is very low. Although the pool size of tropical forests was very large initially, the large output term, no inputs, and very small positive internal changes mean that the net change is strongly negative and the pool size is dropping rapidly. This is exactly how scientists calculate the rate of loss of rainforests, as was presented in Units 3 and 4. To understand element cycles, biogeochemists also calculate residence times of materials in the pool, which is the average amount of time that the element or material spends in the pool before being removed. If there are 100 trees in a forest, and 5 new trees are added and 5 are removed each year, then the residence time is equal to the pool size divided by the flux rate (in or out, it doesn’t matter), or (100 trees) / (5 trees per year) = 20 years; in this case the 20 years is also the average life span of the tree). If the residence time is very short that often means that the component is converted to something else quickly or that it is very reactive (see also Figure 10.7 the Hydrologic Cycle section below). Thus, mass balance equations are useful because they allow one to make predictions about the impact of changes on resource flow and changes in the amounts of materials or elements we have on Earth.
What
are some of the most important chemical reactions on Earth?
Six key elements make up ~95% of
all living organisms: carbon (C), hydrogen (H), oxygen (O), nitrogen (N),
phosphorus (P) and sulfur (S). There are also a number of other elements
important to living organisms such as potassium (K), calcium (Ca), and magnesium
(Mg). Chemical oxidation and reduction reactions involving these key elements
are crucial for life because they link the chemical, biotic, and geologic
systems together. These
reduction-oxidation (redox) reactions are usually
mediated by organisms and especially bacteria, who gain energy from the
exchanges of electrons (Figure 10.2).
For example, oxidation is removal of electrons, which releases energy
(e.g. fire), or rusting where the oxidation of iron from Fe2+
à Fe3+
releases energy that some bacteria can use to grow.
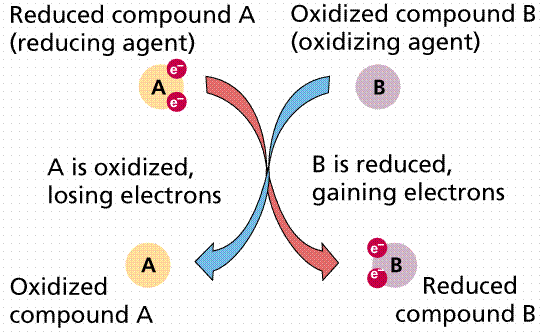
Figure 10.2
Redox
reactions
http://www.emc.maricopa.edu/faculty/farabee/BIOBK/redox.gif
In redox
reactions there must be a compound that can donate or release electrons, plus
one that can accept electrons. For
aerobic, heterotrophic organisms like us, the oxidation and reduction reactions
of photosynthesis and respiration are arguably the most important chemical
reactions. In photosynthesis the CO2
from the atmosphere serves as the electron acceptor. Plants can use light energy
and take inorganic compounds, such as CO2 and H2O, and
create organic matter such as sugar compounds, and in the process release
gaseous O2. The corresponding oxidation reaction is aerobic
respiration, where plants as well as other organisms consume O2 (the
electron acceptor) as they break down sugars (the electron donor) which
releases energy and CO2. This set of reactions is crucial to life
because heterotrophs consume the sugars and the
oxygen that the autotrophs produce in photosynthesis;
in the equation below, photosynthesis is the reaction proceeding from left to
right, and respiration is the reaction proceeding backwards from right to left.
6 CO2
+ 12 H2O ßà C6H12O6 + 6 O2
Nitrogen gas makes up 78% of the
atmosphere on Earth. However, this N is not in a form that is biologically
available to most organisms. Nitrogen fixation is a key chemical reaction
produced by bacteria, which can take inorganic N2 gas from the
atmosphere and convert it to organic-N compounds that organisms can use. Denitrification is the opposite reaction to N fixation
(like the photosynthesis and respiration reactions) and is important for
returning N2 gas to the atmosphere. Denitrifying organisms can
reduce nitrate back to N2 gas and release energy in the process.
2 N2
+ 6 H2O ßà 4 NH3 + 3 O2
The byproduct of nitrogen
fixation (ammonium, NH3) is critical to life because ammonium is a
key component of amino acids, which are the building blocks of DNA and proteins
(see Unit 3). Amino acid synthesis uses ammonium, water, and CO2 to
form amino acids and O2.
2 NH3
+ 2 H2O + 4 CO2 ßà 2 CH2NH2COOH + 3 O2
Bacterial and fungal
decomposition reactions are important because they allow organic carbon compounds
to be broken down and used as energy sources. If decomposition did not occur,
the Earth would quickly be overrun by organic material and biogeochemical
cycling would eventually cease – in other words, there would be no new source
of nutrients like N and P for the autotrophs to use
in photosynthesis. Once the oxygen is used up in aerobic respiration, bacteria
use alternate electron acceptors to facilitate anaerobic decomposition, which
is one of the most primitive metabolic pathways and was important to the
evolution of life. Organisms found in the early ocean would have used this
pathway as an energy source. Today organisms in anoxic environments, such as
lake and ocean sediments, wet soils, wetlands, and even some eutrophic estuaries, use this pathway to break down organic
compounds.
2 CH2O + electron acceptor ßà CO2
or CH4
The carbonate equilibria reaction is another key biochemical reaction,
which makes it possible for the ocean to absorb CO2 from the
atmosphere and makes carbonate available for aquatic organisms to incorporate
into their shells. Without this reaction, Earth probably would be a runaway
greenhouse planet like Venus; however, during the out-gassing period in Earth’s
history, rain was responsible for scavenging the huge amounts of CO2
from the atmosphere and the reaction below was responsible for storing the CO2
in the form of carbonate (HCO3-).
H2O
+ CO2 ßà H2CO3
ßà H+
+ HCO3-
The weathering of feldspar and other silicate minerals in rocks is also important to biological systems because it makes K, P, and other mineral nutrients available for uptake. This process is an example of how the geologic, chemical, and biotic cycles are linked together.
2 KAlSi3O8 + 2(H+ +
HCO3-) + H2O ßà Al2Si2O5(OH)4
+ 4 SiO2 + 2K+ + 2 HCO3-
How
are human activities affecting nutrient cycling?
Nitrogen (N) and phosphorus (P) availability have strong impacts on the functioning of the biosphere because these elements are key components of biological reactions, enzymes, DNA, and ATP. Both N and P cycles have rapid turn over rates (short residence times) in most systems and are connected to large global pools. Soils and rocks are the most important reservoir for P, with rock weathering being the main mechanism to release “new” P for use in biological systems. P is also supplied by the decomposition of organic matter. The P cycle is unique in that it does not have a significant atmospheric component like the hydrologic, C and N cycles. Organic forms of P and N are generally recycled efficiently in many ecosystems. However, the most important reservoir for N is the atmosphere, which contains 3.9 x 1021 g N, making up 78% of the atmosphere, mostly in the form of N2 gas. The N cycle is complex because there are many chemical forms of N (Figure 10.3) including, organic-N, nitrate (NO3-), ammonium (NH4+); and a number of gases such as, N2, N2O, NO and NO2 (together, also known as NOx).
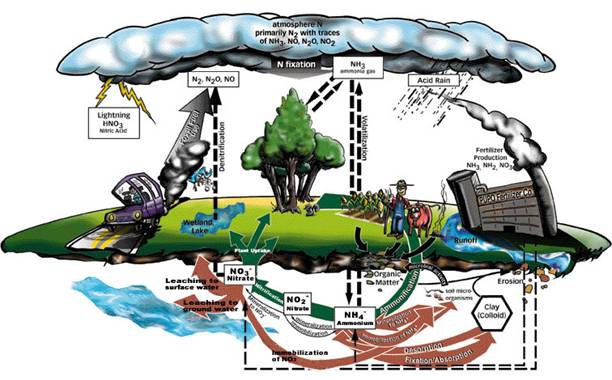
Figure 10.3
Forms of nitrogen
http://www.hawaii.edu/malama/teachers/TLunsford/N_cycle/Nitrogen_cycle_diagram.jpg
The most abundant form of N, dinitrogen gas (N2) is not readily available for most organisms to use due to the strength of the triple bond holding the two N atoms together. Therefore, N2 must be broken down and converted into other forms of N, such as NH3 through N-fixation, to be useful in biological systems. Historically this process has been carried out by certain microbes, and to a small degree, lightening. However, in the 1930’s an industrial chemical process of N-fixation known as the Haber-Bosch process was developed for use in agricultural chemical fertilizers. This process, as well as the increasing use of fossil fuels that release N compounds into the atmosphere, have dramatically altered the global N cycle. For example, from 1900 to 2000 human inputs of N have increased from 20 to 170 Tg N yr–1. (Note that 1 Tg or teragram is equal to 1,000,000,000 kilograms!) In the next 50 years, these inputs to the global N cycle are expected to continue to increase to 275 Tg N yr–1. Currently only 40% of all newly fixed N deposited on the Earth’s surface each year comes from natural biological and chemical processes, whereas 60% is derived from human sources. This is an unprecedented change on a global scale!
Once N is fixed into a biologically useful form, it can go through many biochemical pathways (Figure 10.4). Fixed N such as NO3 and NH4 can be taken up by plants and converted to organic N in plant tissues through photosynthesis. Organic-N in discarded plant material (litter) can then be mineralized (decomposed) or recycled into NH4 for reuse by plants, bacteria, or fungi. Nitrate can go through the biological denitrification process by bacteria, which converts NO3 back into N2 gas. Nitrate and NH4 can also be taken up by nitrifying bacteria and converted to NO or N2O (NOx) gases for release into the atmosphere.
In addition to NOx gases released to the atmosphere by nitrifying bacteria, the burning of fossil fuels is a major source of NOx in the atmosphere. Once NOx are in the atmosphere, these compounds can be transported long distances and may react with other compounds like ozone, or combine with rainwater to form nitric acid, or acid rain. These atmospheric compounds may be re-deposited on the Earth’s surface as atmospheric deposition to be taken up by plants or microbes. Nitrogen deposition (often in the form of nitrate) not absorbed by terrestrial ecosystems may leach into streams, rivers, and groundwater. This excess nitrogen deposition can severely impact terrestrial and aquatic ecosystems by altering species composition, increasing acidity, causing eutrophication which consumes oxygen and results in “dead zones” in lakes and oceans, and causing the leaching loss of other essential nutrients like calcium.

Figure 10.4
Global N cycle
Adapted from Biogeochemistry: An
Analysis of Global Change by William H. Schlesinger
How
does the global carbon cycle work?
Let’s follow an atom of carbon (C)
to better understand how C cycles, or moves from one form of C to another and
among the different C pools on Earth. Carbon is found in many forms on Earth;
as inorganic-C such as bicarbonate and carbonate in rocks, as organic-C like
that in plant and animal tissues, and as a gas, for instance carbon dioxide (CO2),
methane (CH4) or carbon monoxides (CO). In addition, there are many
paths that a C atom can take as it cycles on Earth; therefore, this example
illustrates only one possible pathway.
Assume that an atom of C is in
sedimentary rock that has been subducted near a
volcano. Tectonic activity builds pressure in the volcano, and localized areas
of rock melt and begin to release gases from the crater of the volcano. Our C
atom is oxidized and released into the atmosphere as CO2 (Figure
10.5). This CO2 molecule spends time in the atmosphere floating
around and absorbing long wave radiation that the Earth emits to space as part
of Earth’s radiative energy balance. Because the CO2
molecule is absorbing long wave radiation and preventing the radiation from escaping into space,
this warms the atmosphere. Eventually, the molecule of CO2 floats
over a forest and is taken up into a tree leaf. The tree uses light energy and
the molecule of CO2 in photosynthesis and makes it into sugar, or
organic-C.
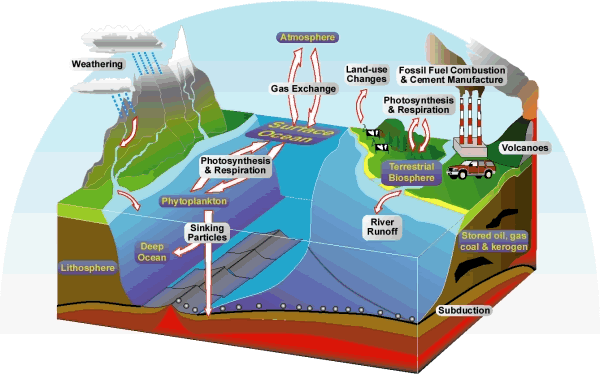
Figure 10.5
Global C cycle
http://www.uwyo.edu/terra/images/c_ccycle_lg.gif
Recall that photosynthesis and
respiration are arguably the most important chemical reactions on Earth because
together these reactions convert inorganic-C in the oxidized form of CO2,
to the reduced form of organic-C in living tissue. Respiration then converts
organic-C back to inorganic-C, which is released from the breakdown of these
tissues for energy. This set of biological reactions directly or indirectly
provides energy for most life forms on Earth, and links together the C
biogeochemical cycle.
Going back to our C atom, the new organic sugar molecule
in the leaf is then used to make a fruit. A hungry insect eventually finds the
new fruit, which contains our C atom. The insect uses the organic-C for energy
and incorporates it into its tissues. When the insect dies it falls onto the
forest soil and microbes begin decomposing the tissue. Soil microbes take up the
molecule of organic-C and use it for energy. They break down the organic-C,
oxidizing it in the process, which releases it back to the atmosphere as CO2.
Once again the CO2 molecule floats around the atmosphere absorbing
long wave radiation and warming the atmosphere. Eventually this molecule makes
its way to the ocean where it is mixes with the surface water. Phytoplankton in
the water, take up the molecule of CO2 and use it to again make
organic-C, which it incorporates into its tissues. The phytoplankton are
then eaten by zooplankton, which use the C to make a carbonate shell.
Eventually the zooplankton die and sink to the bottom of the ocean, where over
time the carbonate shell becomes part of the sedimentary rock to begin the C
cycle again.
Most of C on Earth is buried in
sedimentary rock as organic-C and carbonate. This C can be stored in the Earth
for long periods of time. However, some of it can be released by rock
weathering, from volcanic eruptions, or from the extraction of fossil fuels,
which are then burned (or oxidized) and put into the atmosphere. During the
carboniferous period in Earth’s history, large deposits of organic-C from
plants were stored in the Earth’s crust, where over time they turned into coal,
oil, and natural gas reserves. Today, humans are tapping into this large store
of energy. However, in the process we are also altering the balance of the C
cycle by significantly increasing the amount of CO2 and other
greenhouse gases in the atmosphere.
In addition to the burning of
fossil fuel, which is altering the composition of the atmosphere, humans are
impacting the C cycle through land use change. By converting forests to
agricultural land, clear-cutting and burning tropical forests, and by
converting rural lands to urban areas, humans are decreasing the sequestration
capacity of the Earth and increasing the amount of C in the atmosphere.
Scientists have determined that in the last 650,000 years or more, the
concentration of CO2 in the atmosphere has never risen above 300ppm.
However, since the industrial revolution, the concentration of CO2
in the atmosphere has been steadily increasing and today is ~387ppm. In the
next 50-100 years we expect this to continue to increase up to more than
700ppm, which will have important consequences for the radiative
balance of the Earth and global climate.
Why
is the hydrologic cycle important?
Earth is unique in that it is
the only planet in our solar system with large quantities of liquid water,
which is necessary to support life and maintain the Earth’s radiative
energy balance. The movement of water on Earth transfers heat energy from the
warm tropics to the cold poles through the mechanisms of evaporation, vapor
transport, precipitation, and run off (Figure 10.6). This movement of water
around the globe is the largest movement of a chemical substance on Earth and
is strongly responsible for regulating climate and determining vegetation
patterns. The global hydrologic cycle is also strongly connected to the carbon
and nutrient cycles, as water is largely responsible for plant growth, rock
weathering, and nutrient transport around the world.
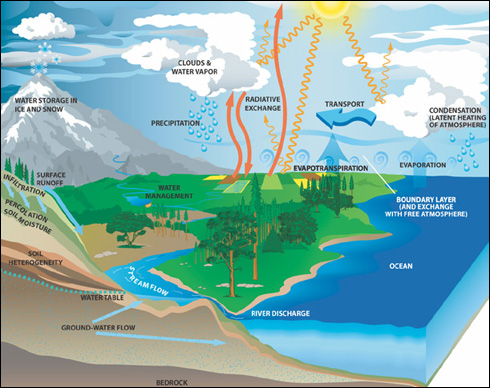
Figure 10.6
Global hydrologic cycle
http://science.hq.nasa.gov/oceans/system/water.html
The global hydrologic cycle has four main fluxes or pathways, which include: evaporation from the ocean and land surfaces, vapor transport by the atmosphere, precipitation onto the land and ocean surfaces, and runoff of freshwater back into the oceans. The Equatorial region receives the majority of the radiation from the Sun, which heats up the surface of the land and the surrounding tropical seas. This tropical heating fuels the general circulation of the atmosphere and controls regional hydrology and climate. As the tropical ocean water heats up, it evaporates and in the process decreases the temperature of the surface by latent heat flux. The warm vapor is then transported away from the equator by trade winds and the rotation of the Earth. As the clouds cool, they condense and form precipitation, which is deposited on the land and back onto the ocean surface. Eventually, excess freshwater deposited on the land makes its way back into the ocean from runoff, to start the process again. Note that additional fluxes, such as ocean currents, also move heat and materials across the globe.
When water is on the land
surface, there are additional pathways that can delay the return of freshwater
to the oceans. For example, freshwater can be deposited in snow and ice
(glaciers and sea ice), in deep groundwater, stored in soils and freshwater
lakes and reservoirs, or diverted and used for human consumption. In addition, evapo-transpiration on land (evaporation of water directly
from the soil plus transpiration of water through plants to the atmosphere) and
the recycling of precipitation are important for many vegetated regions. For
example, in the Amazon basin only 46% of all rainfall originates from long
distance transport from the ocean, whereas 54% of all precipitation that falls
in the basin originates from evapo-transpirative
recycling from within the basin! Eventually though, rainfall in the basin makes
it’s way back to the ocean by way of the Amazon river, which transports 20% of
the worlds freshwater to the oceans.
The cycling of water through the
hydrologic cycle links the atmosphere, oceans, and terrestrial systems, and is
responsible for much of the climate variability on Earth. Changes in Earth’s
hydrologic cycle can have large impacts on people and ecosystems because the
distribution of rainfall is the most important factor influencing plant growth,
which produces the energy that the rest of the planet relies upon. Humans have
altered precipitation and the runoff patterns of rivers around the globe. For example,
more than 77% of river discharge in the northern hemisphere is regulated by
dams, which have huge impacts on the runoff rate back into the ocean. Land use
changes such as deforestation, and the diversion of rivers for agriculture and
drinking water, also have significantly impacted precipitation and runoff
rates. Climate change is expected to have considerable impacts on the global
hydrologic cycle, with most models predicting changes in global precipitation
patterns around the world – some places will have more and others less rain.
This could have large consequences for the Earth’s growing human population,
which currently has freshwater shortages in many regions. In addition, climate
change is also expected to significantly impact sea levels and ocean
circulation from the melting of polar glaciers and ice caps, which has the
potential to substantially alter climate and precipitation patterns around the
globe.
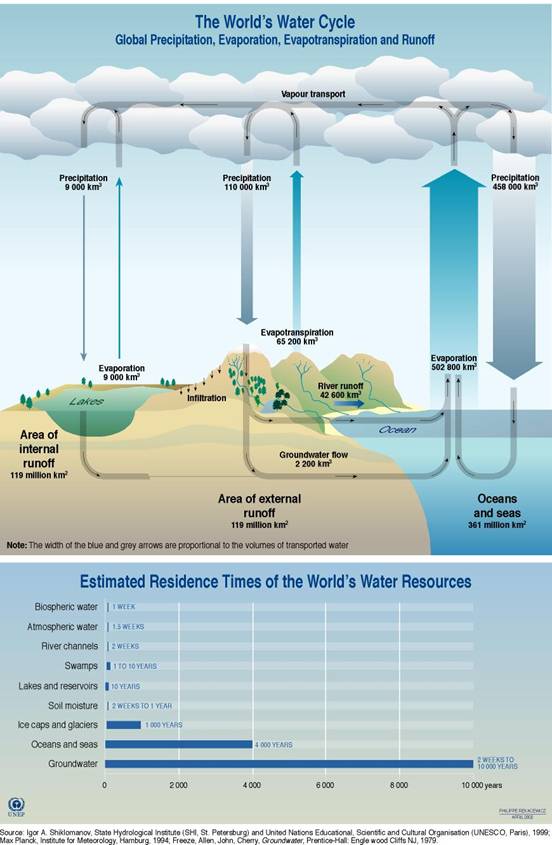
Figure 10.7
Global hydrologic cycle and
residence times of the worlds water resources
http://maps.grida.no/go/graphic/world_s_water_cycle_schematic_and_residence_time
What feedbacks result
from human alteration of Earth’s biogeochemical cycles?
Since biogeochemical cycles are
inextricably linked to one another, and also linked to the climate of Earth,
changes in one cycle have inevitable consequences for other cycles. Most of
these changes occur due to feedback effects. A feedback in a system can be
understood as a proportion, or function, of the output of the system being
passed back into the input variable. For example, the burning of fossil fuels
is increasing the amount of CO2 in the atmosphere, which then warms
the global climate. In polar-regions like the boreal forest, huge amounts of C
are stored in the permanently frozen soil beneath the boreal forests. As
temperatures increase, the permafrost begins to melt and releases the stored C.
This C released from the melting of the permafrost adds to the CO2
in the atmosphere and causes a positive feedback loop, which further increases
global warming (Figure 10.8).
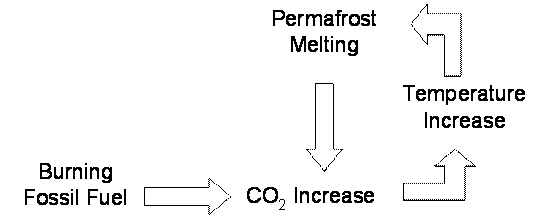
Figure 10.8
Positive feedback loop
In addition to altering other
cycles, anthropogenic changes in biogeochemical cycles often feedback and negatively
impact humans and the ecosystems that they depend upon. One example of this
negative feedback is apparent in freshwater and marine dead zones, or large
areas in the world’s lakes and oceans that become low (hypoxic) or devoid
(anoxic) of oxygen and therefore can not support fish or other aquatic life.
Each year in the
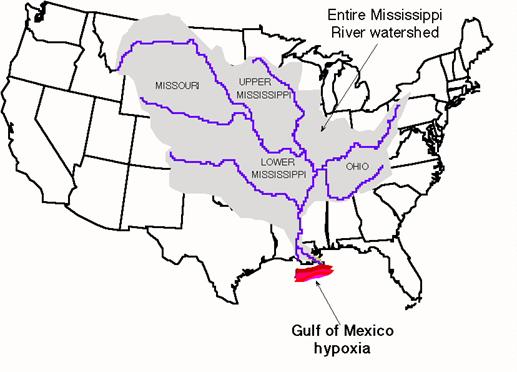
Figure 10.9
Hypoxia in the
http://researchnews.osu.edu/archive/hypoxia.jpg
In an attempt to increase crop
yield, farmers often use excess chemical fertilizers of N and P on soils, which
then leach into groundwater, streams, and rivers. The level of pollutant
fertilizers has more than doubled in the
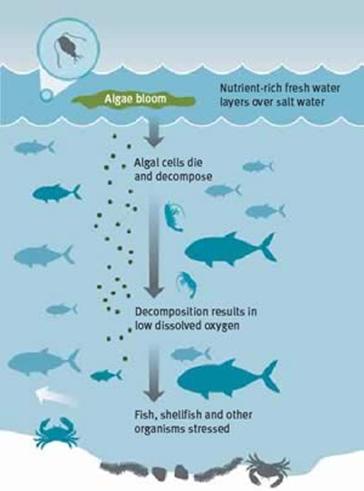
Figure
10.10
Mechanism
for hypoxia in the Gulf of Mexico
http://www.lacoast.gov/watermarks/2004-09/1hypoxia/hypoxia.jpg
Because the most energetically
efficient decomposition reactions require oxygen, microbes first use up all the
available oxygen to decompose the large quantities of detritus that is
available, before they move on to anoxic decomposition reactions and this
creates large hypoxic zones in the Gulf. Hypoxia kills fish, shellfish, and
other aquatic organisms, and has severely damaged the fishing and shrimp
farming industries in the region. In addition to hypoxia, pollutant fertilizers
are also responsible for red tides, or dinoflagellate
blooms, which produce large quantities of toxins that make shellfish like clams
and oysters unfit for human consumption, cause neurological damage to aquatic
mammals, and cause respiratory problems in humans.
Last updated: 10/23/2006 5:17 PM